

An intentionally designed, high quality product that drives patient satisfaction.
If you are a potential patient, please learn more about the product here.
Jeuveau is a 900 kDa neurotoxin designed with purpose in California. Our unique manufacturing combines vacuum-dried processing with proprietary
resulting in ≥95% product purity and a
diffusion profile that supports results as you intend them.2,9


Jeuveau utilizes the entire 900kDa complex. Accessory proteins are active and may contribute to the efficacy.8

Our HI-PURE™ Technology allows the purification of the toxin with at least
95% purity.1
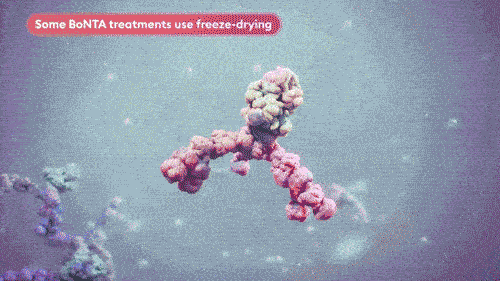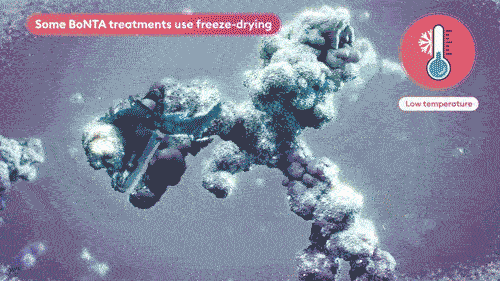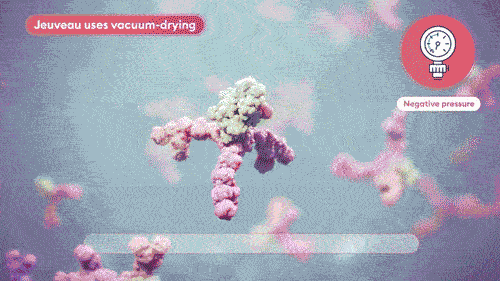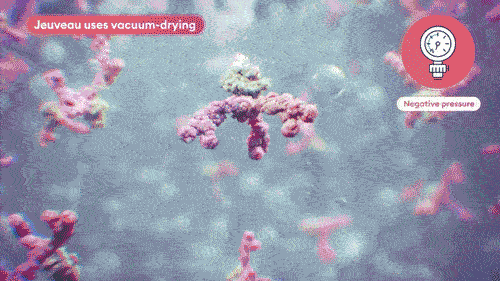
Vacuum-dried avoids crystal formation and minimizes product impurities, maintaining the integrity of the toxin.8
When used in conjunction with intramuscular injection for moderate to severe glabellar lines,1,4 Jeuveau offers:
*87% of injectors consider Jeuveau to be a precise product when injected intramuscularly.8
Jeuveau demonstrated a proven safety profile in 5 clinical studies, all part of the Transparency Clinical Program:
In the 2 U.S. pivotal phase III studies, 68% and 70% of Jeuveau patients with moderate to severe frown lines had a 2-grade or better physician- and patient-rated improvement (none or mild frown lines) at Day 30, compared to 1% for placebo.
* Jeuveau demonstrated non-inferiority to BOTOX® Cosmetic in the treatment of moderate to severe glabellar lines in adults based on a GLS score of 0 or 1 at maximum front on Day 30 by investigator assessment.
.png)

We’re so confident in our results, we put Jeuveau to the test against the legacy neurotoxin brand, BOTOX® Cosmetic.5
Evolus conducted the largest head-to-head aesthetic phase III study and found that Jeuveau demonstrated non-inferiority to BOTOX® Cosmetic in the treatment of moderate to severe glabellar lines in adults based on a GLS score of 0 or 1 at maximum frown on Day 30 by investigator assessment.**
**A non-inferiority study is conducted to show that the effect of a treatment is not worse than the original treatment.
GLS score of 0 or 1 at maximum frown on day 30 by investigator assessment.


*Investigator and subject assessment of GLS ≥1-point improvement at maximum frown at days 2, 14, 30, 90, 120, and 150.
94.5% of Jeuveau patients report being satisfied with their results, enjoying smoother frown lines in as little as two days* that last for up to four months.5†
*About 50% of people begin showing improvement after two days (≥1-grade improvement by physician assessment). Most people (~69%) achieve complete results by day 30 (≥2-grade improvement by physician and patient assessments). †On average, Jeuveau patients receive 3 treatments a year.






































































In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.

In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician-and patient-rated improvement in frown lines at Day 30.3
In a study conducted in Europe and Canada, 54% of Jeuveau patients had a ≥1-grade improvement in frown lines at Day 2 based on physician assessment compared to 12% for placebo (secondary endpoint).2 In US studies, 46% and 56% had a ≥1-grade improvement in frown lines at Day 2 based on physician-assessment compared to 8% and 17% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be considered clinically meaningful.
In US studies, 68% and 70% of Jeuveau patients had a 2-grade or better physician- and patient-rated improvement in frown lines at Day 30, compared to 1% for placebo.3
In a study conducted in Europe and Canada, 58% of Jeuveau patients had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 13% for placebo (exploratory endpoint).2 In US studies, 65% and 57% had ≥1-grade improvement in frown lines at Day 120 based on physician assessment, compared to 8% and 13% for placebo (exploratory endpoint).3 Results based on a 1-grade improvement may not be clinically meaningful.
Start setting up your MyEvolus account and see all that Evolus can offer you today.
Exclusively licensed and manufactured for: Evolus, Inc., 520 Newport Center Drive, Suite 1200, Newport Beach, CA 92660
©2025 Evolus, Inc. All rights reserved. All trademarks are the property of their respective owners.
*Your Club Evolus membership covers 20 units of Jeuveau per treatment, with a minimum of 90 days between each treatment. Units do not accrue if you wait more than 90 days between treatments. You will pay your provider directly for any additional treatments you receive. Cannot be combined with any other offer. Club Evolus members will be automatically opted out of Evolus Rewards™ upon enrollment. You can manage your Club Evolus account and payment method through your club.evolus.com login. If you cancel within the first three months a $100 cancellation fee will apply. Your Club Evolus membership is non-transferable and payments are non-refundable.
WARNING: DISTANT SPREAD OF TOXIN EFFECT: The effects of all botulinum toxin products,including JEUVEAU, may spread from the area of injection to produce symptoms consistent withbotulinum toxin effects. These symptoms have been reported hours to weeks after injection.Swallowing and breathing difficulties can be life threatening and there have been
reports of death.JEUVEAU is not approved for the treatment of spasticity or any conditions other than glabellarlines.
JEUVEAU is contraindicated in individuals with known hypersensitivity to any botulinum toxinpreparation or to any of the components in the formulation, and in the presence of infection
at theproposed injection site(s).
Postmarketing safety data from other approved botulinum toxins suggest that botulinum toxineffects may be observed beyond the site of local injection. The symptoms are consistent
with themechanism of action of botulinum toxin and may include asthenia, generalized muscle weakness,diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence, blurred
vision and breathingdifficulties. These symptoms have been reported hours to weeks after injection. Swallowing andbreathing difficulties can be life threatening and there have been
reports of death related to spreadof toxin effects. In unapproved uses, including upper limb spasticity in children and approvedindications, symptoms consistent with spread of toxin
effect have been reported at dosescomparable to or lower than the maximum recommended total dose. JEUVEAU is not approved forthe treatment of spasticity or any conditions other
than glabellar lines. Patients or caregivers shouldbe advised to seek immediate medical care if swallowing, speech or respiratory difficulties occur.
The potency Units of JEUVEAU are specific to the preparation and assay method utilized. They are notinterchangeable with other preparations of botulinum toxin products and, therefore,
units ofbiological activity of JEUVEAU cannot be compared to nor converted into units of any otherbotulinum toxin products assessed with any other specific assay method.
Serious adverse reactions, including excessive weakness, dysphagia, and aspiration pneumonia, withsome adverse reactions associated with fatal outcomes, have been reported in
patients who receivedbotulinum toxin injections for unapproved uses. In these cases, the adverse reactions were notnecessarily related to distant spread of toxin, but may have resulted
from the administration ofbotulinum toxin products to the site of injection and/or adjacent structures. In several of the cases,patients had pre-existing dysphagia or other significant
disabilities. There is insufficient informationto identify factors associated with an increased risk for adverse reactions associated with theunapproved uses of botulinum toxin products.
Serious and/or immediate hypersensitivity reactions have been reported for botulinum toxinproducts, including anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If suchreactions occur with JEUVEAU, discontinue use of JEUVEAU and immediately institute appropriatemedical therapy.
There have been reports following administration of botulinum toxins of adverse events involving thecardiovascular system, including arrhythmia and myocardial infarction, some with
fatal outcomes.Some of these patients had risk factors including pre-existing cardiovascular disease. Use cautionwhen administering to patients with pre-existing cardiovascular
disease.
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, orneuromuscular junction disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) were
excluded from the JEUVEAU clinical studies. Patients with neuromuscular disorders may be atincreased risk of clinically significant effects, including generalized muscle weakness,
diplopia, ptosis,dysphonia, dysarthria, severe dysphagia and respiratory compromise from typical doses of JEUVEAU.
Treatment with botulinum toxin products, including JEUVEAU, can result in swallowing or breathingdifficulties. Patients with pre-existing swallowing or breathing difficulties may be more
susceptible tothese complications. In most cases, this is a consequence of weakening of muscles in the area ofinjection that are involved in breathing or oropharyngeal muscles that
control swallowing orbreathing. (See Boxed Warning for Spread of Toxin Effect)
Caution should be used when JEUVEAU is used in the presence of inflammation at the proposedinjection site(s) or when excessive weakness or atrophy is present in the target muscle(s). Cautionshould be used when JEUVEAU treatment is used in patients who have marked facial asymmetry,ptosis, excessive dermatochalasis, deep dermal scarring, thick sebaceous skin or
when subjects donot respond to 20 Units of botulinum toxin (e.g. the inability to substantially lessen glabellar lineseven by physically spreading them apart). Do not exceed the
recommended dosage and frequency ofadministration of JEUVEAU.
Dry eye has been reported with the use of botulinum toxin products in the treatment of glabellarlines. Reduced tear production, reduced blinking, and corneal disorders, may occur with
use ofbotulinum toxins, including JEUVEAU. If symptoms of dry eye (e.g., eye irritation, photophobia, orvisual changes) persist, consider referring patient to an ophthalmologist.
JEUVEAU contains albumin, a derivative of human blood. Based on effective donor screening andproduct manufacturing processes, it carries an extremely remote risk for transmission of viraldiseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) is also consideredextremely remote. No cases of transmission of viral diseases or CJD have ever been
reported foralbumin.
The most frequently reported adverse reactions (≥ 1% and placebo) following injection of JEUVEAUwere headache (12%), eyelid ptosis (2%), upper respiratory tract infection (3%), and
white blood cellcount increase (1%).
No formal drug interaction studies have been conducted with JEUVEAU. The potential for certaindrugs to potentiate the effects of JEUVEAU warrant consideration given the potential
risks involved and should be used with caution, including: aminoglycosides or other agents interfering withneuromuscular transmission, anticholinergic drugs, botulinum neurotoxin
products, and musclerelaxants.
The limited available data on JEUVEAU use in pregnant women are insufficient to inform a drugassociated risk of adverse developmental outcomes.
There are no data on the presence of JEUVEAU in human or animal milk, its effects on the breastfedinfant, or its effects on milk production.
Safety and effectiveness in pediatric patients have not been established.
JEUVEAU is an acetylcholine release inhibitor and a neuromuscular blocking agent indicated for thetemporary improvement in the appearance of moderate to severe glabellar lines
associated withcorrugator and/or procerus muscle activity in adult patients.
Please note that this information is not comprehensive. For moreinformation about JEUVEAU, see the full Prescribing Information including BOXED WARNING, and Medication Guide.
To report side effects associated with use of JEUVEAU, please call 1-877-EVOLUS1 (1-877-386-5871). You are encouraged to report side effects of prescription drugs to FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
US-JUV-2500118